Introduction:
Immune thrombocytopenic purpura (ITP) is a diagnosis of exclusion that is characterized by isolated thrombocytopenia with a generalized purpuric rash. If there is a known case for the ITP, it is referred to as secondary ITP. Secondary ITP may often be caused by drugs, systemic immunologic, or infectious diseases. Covid-19 is a disease caused by the SARS-CoV-2 virus, which caused a pandemic in 2020, affecting hundreds of millions of people worldwide. There have been several different hematologic conditions associated with this viral infection, including deep vein thromboses, pulmonary emboli, and many patients with ITP induced by Covid-19. With several case reports and case series available on these patients, the specifics behind the strength of the association, mortality of the patients, and their comorbidities have not been previously explored.
Methods:
Using the national inpatient sample from the years 2019-2020, we analyzed adult hospitalized patients with ITP and Covid-19 based on their International Classification of Diseases - 10 clinical modification codes. We calculated the odds ratios for patients with Covid-19 being diagnosed with ITP compared to other hospitalized patients. Additionally, we calculated the odds of several complications, treatments, as well as concurrent conditions in patients with Covid-19 and ITP. The complications measured were epistaxis or hemoptysis, intracranial hemorrhage (ICH), non-intracranial hemorrhage (nICH), deep vein thrombosis (DVT), pulmonary embolism (PE), and death. Treatments measured were transfusion with either red blood cells (RBC) or platelets. The concurrent conditions evaluated were systemic lupus erythematosus (SLE), hepatitis C and heparin-induced thrombocytopenia (HIT).
Results
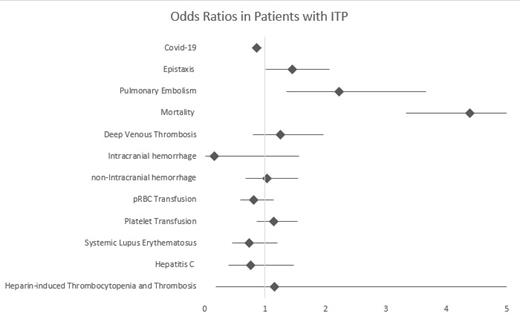
Out of the 115,150 hospitalized patients with ITP included in the database, 0.02% of them were diagnosed with Covid-19. The odds of a patient with Covid-19 being diagnosed with ITP were lower than those for other hospitalized patients (OR=0.860 [0.787-0.941], p=0.001). The mean age for patients with ITP diagnosed with Covid-19 was 64.1 compared to 60.9 in patients without Covid-19 (p=0.001). Hospitalized patients with ITP who were also diagnosed with Covid-19 were at a higher risk of developing epistaxis (OR: 1.45 [1.01-2.07], p=0.042), pulmonary embolism (OR: 2.22 [1.35-3.66], p=0.002), and death (OR: 4.39 [3.33-5.80], p<0.001) compared to those without Covid-19. They were not at higher odds of developing a DVT (OR: 1.25 [0.798-1.97], p=0.325), ICH (OR: 0.158 [0.0160-1.56], 0.114) or nICH (OR: 1.03 [0.678-1.55], p=0.905). They were not significantly more or less likely to get transfused with RBC (OR: 0.816 [0.585-1.14], p=0.231) or platelets (OR: 1.14 [0.865-1.53], p=0.337). The ITP patients with concurrent Covid-19 were also not more likely to suffer from SLE (OR: 0.737 [0.453-1.20], p= 0.222), Hepatitis C (OR: 0.760 [0.392-1.47], 0.416), or HIT (OR: 1.16 [0.181-7.49], p=0.872).
Conclusion
Hospitalized patients with Covid-19 were at a lower risk of being diagnosed with concurrent ITP than other hospitalized Covid-19 patients. Those with ITP who also had Covid 19 had significantly higher odds of complication of bleeding with epistaxis or hemoptysis as well as a higher risk of pulmonary embolism and death. However, they were not at higher risk of hemorrhage elsewhere or DVT. They were not treated with transfusions differently and having SLE, hepatitis C or HIT did not place hospitalized patients with ITP at a higher risk of developing Covid-19.
Disclosures
Liles:Annexon Biosciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Alpine Immune Sciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Abbvie: Other: Clinical trial activity (Principal investigator or sub-investigator); Astex Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); Baxalta: Other: Clinical trial activity (Principal investigator or sub-investigator); BeiGene: Other: Clinical trial activity (Principal investigator or sub-investigator); Bioverativ: Other: Clinical trial activity (Principal investigator or sub-investigator); CSL Behring: Other: Clinical trial activity (Principal investigator or sub-investigator); Celgene: Other: Clinical trial activity (Principal investigator or sub-investigator); Delta-Fly Pharma: Other: Clinical trial activity (Principal investigator or sub-investigator); Exact Sciences: Other: Clinical trial activity (Principal investigator or sub-investigator); Forma Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Global Blood Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Immunovant: Other: Clinical trial activity (Principal investigator or sub-investigator); Incyte: Other: Clinical trial activity (Principal investigator or sub-investigator); Janssen Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); NeoImmuneTech: Other: Clinical trial activity (Principal investigator or sub-investigator); Novartis: Other: Clinical trial activity (Principal investigator or sub-investigator); Novo Nordisk: Other: Clinical trial activity (Principal investigator or sub-investigator); Partner Therapeutics: Other: Clinical trial activity (Principal investigator or sub-investigator); Pharm-Olam: Other: Clinical trial activity (Principal investigator or sub-investigator); Principia Biopharma: Other: Clinical trial activity (Principal investigator or sub-investigator); Salix Pharmaceuticals: Other: Clinical trial activity (Principal investigator or sub-investigator); Sanofi-Aventis: Other: Clinical trial activity (Principal investigator or sub-investigator); Takeda: Other: Clinical trial activity (Principal investigator or sub-investigator); Vifor Pharma: Other: Clinical trial activity (Principal investigator or sub-investigator).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal